28+ bomb calorimeter calculator
Record the room temperature as this will be necessary to calculate the stem correction as discussed. The calorimeter held 2500 kg of.
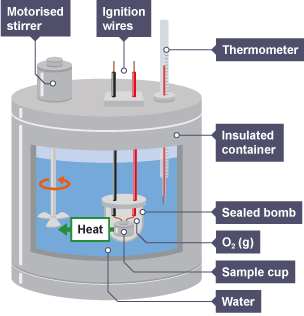
Combustion Bomb Calorimeter Specific Heat Calculation Of The Quantity Of Heat Science Online
Web To calculate the heat capacity of a calorimeter put q -1849 kJ and T 2793 C 2674 C in the equation ii 1849 kJ c 2793 2674 c 1849 kJ119 C c 1554 kJC.
. By knowing the initial mass of the fuel sample the heating value of the sample can be calculated by dividing the heat. Web A bomb calorimeter is a device used to measure the heat of combustion of a substance which is the amount of energy released when a substance is burned. Web A bomb calorimeter is an instrument used to determine the heat emitted from a given quantity of biomass sample combustion and to calculate the HHV of that biomass fuel.
After stirring and waiting for the. It consists of a. Assume no heat is absorbed by the calorimeter no heat is exchanged between the calorimeter and its surroundings and that the specific heat and mass of the.
Web 134 13 kJ. Web In this example we calculate the heat capacity of a bomb calorimeter using constant volume calorimetry given the change in internal energy for a combustion. Web A bomb calorimeter is a tool used to calculate the heat of a reaction at a fixed volume.
Web The heat capacity of the bomb calorimeter was calculated to be 114 kjc by the manufacturer. The change in internal energy ΔE is then measured and called the calculated heat. Web Temperature profile from a bomb calorimeter experiment.
Web Parr Bomb calorimeter and power supply S. Δe c v 3078 kj 088 g 3498 kjg. Feb 04 2020 AP Chemistry.
Web A bomb calorimeter is used to measure the heat created by a sample burned under an oxygen atmosphere in a closed vessel bomb which is sur- rounded. Web qrxn qcalorimeter where qcalorimeter qbomb qwater If the constant volume calorimeter is set up the same way as before same steel bomb same amount. 7255 g of water at 716 C added to a calorimeter containing 5885 g of water at 224 C.
A calorimeter is to be calibrated.
Bomb Calorimetry
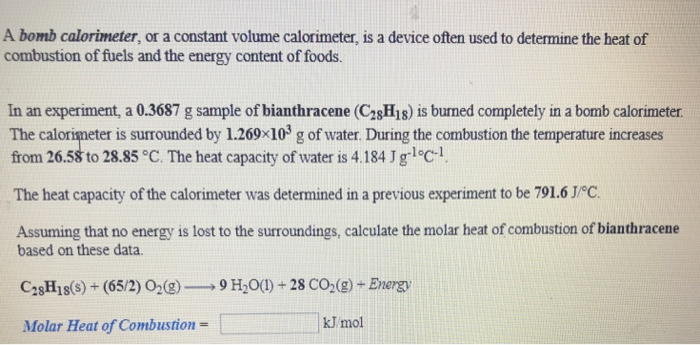
Solved Calculate The Heat Capacity Of The Calorimeter Chegg Com
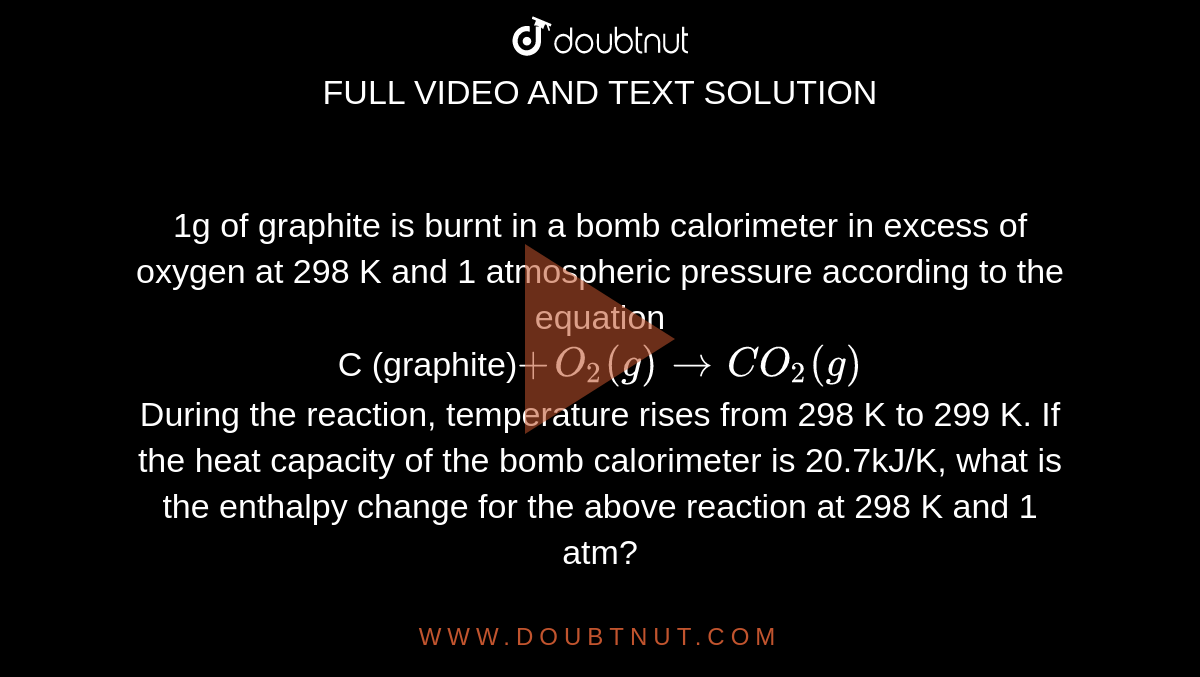
1g Of Graphite Is Burnt In A Bomb Calorimeter In Excess Of Oxygen At 298 K And 1 Atmospheric Pressure According To The Equation C Graphite O 2 G Rarrco 2 G During The Reaction Temperature Rises From
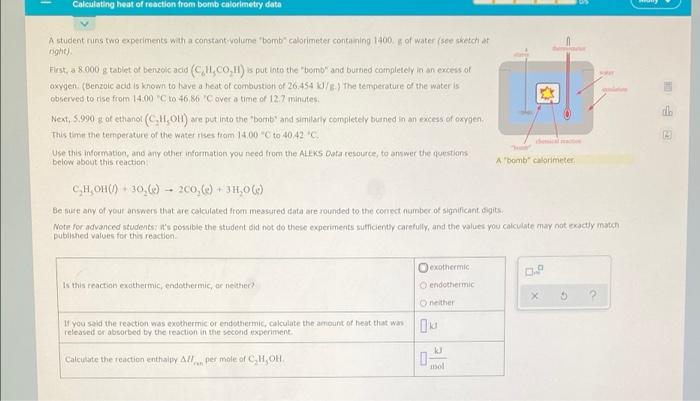
Solved Calculating Heat Of Reaction From Bomb Calorimetry Chegg Com

Types Of Calorimetry Problems I Calculating The Specific
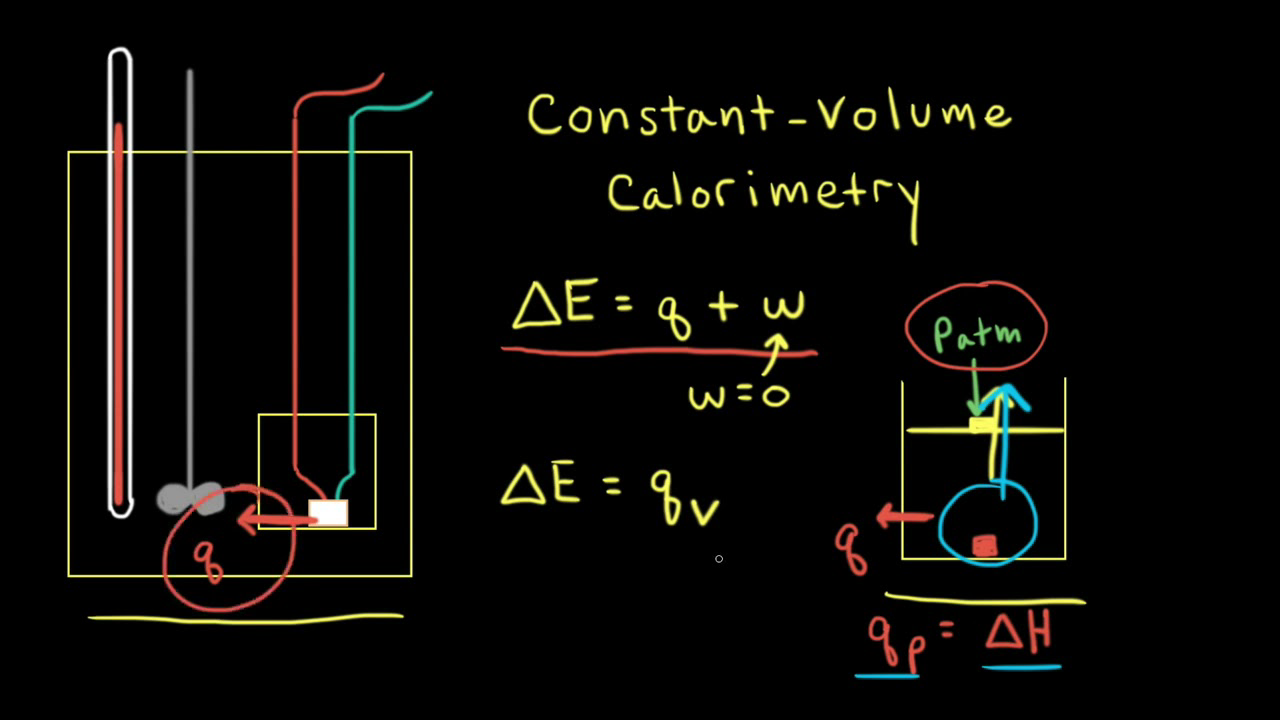
Constant Volume Calorimetry Video Khan Academy

Answered Calculate The Ag Rxn Using The Bartleby

Bomb Calorimeter Equation Calculation What Does A Bomb Calorimeter Measure Video Lesson Transcript Study Com

Chemistry 101 Calculating Heat Capacity Of A Bomb Calorimeter Youtube
Calorific Value Definition Calculating Method Formula And Significance

Calorimeter Bomb
Bomb Calorimetry

Bomb Calorimeter Calculation
Bomb Calorimetry

Solved Question 5 Not Yet Answered Marked Out Of 4 00 Flag Question A0 1326 G Sample Of Magnesium Was Burned In An Oxygen Bomb Calorimeter The Total Heat Capacity Of The Calorimeter Plus

Under Constant Volume Conditions The Heat Of Combustion Of Sucr Pearson Channels
Speaking About Food Metabolism Math And The Calorie